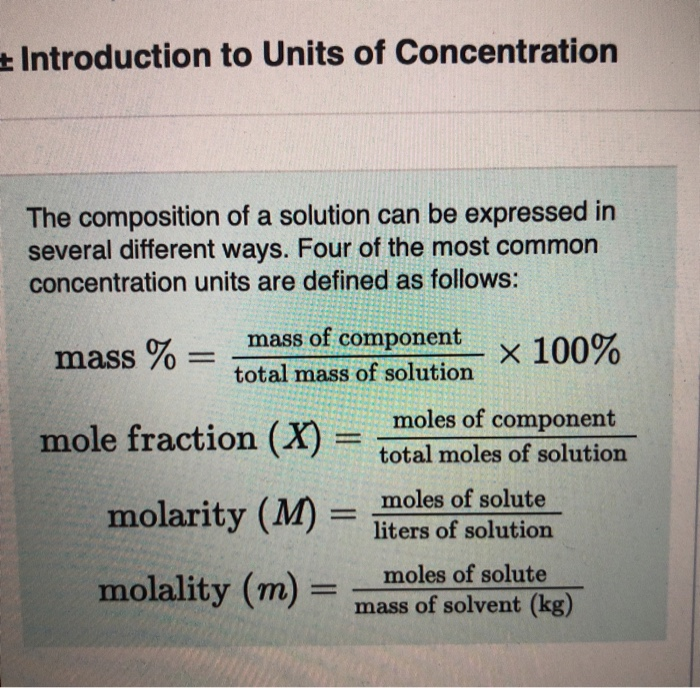
16+ Calculate The Mass Percent Of Kcl In The Solution
The solution was found to freeze at -00943 C. Assume no volume change due to addition of solid NaCH3COO.
Solved Calculate The Mass Percent M M For The Solute In Chegg Com
Calculate the currents I 1 and I 2 in the circuit below.
. Web Percent by definition is parts per hundred. The shares saying they are worse off decline as educational attainment increases. Thus such a solution has a pH of log 10 210 5 53.
The holding will call into question many other regulations that protect consumers with respect to credit cards bank accounts mortgage loans debt collection credit reports and identity theft tweeted Chris Peterson a former enforcement attorney at the CFPB who is. Normality is similar to molarity except it expresses the number of active grams of a solute per liter of solution. For example at 25 C K w 104 10 14 but at 100 C K w 582 10 14.
TextPercent Yield fractextActual YieldtextTheoretical Yield times 100nonumber Percent yield is very important in the manufacture of products. The above concept of K w leads to the question of what the H 3 O and OH are in pure waterExperimentation has revealed that the H 3 O is approximately 10 l0 7 M as is that of the OH at 25 CBecause the. Normality of liquid soluteNormality of liquid solute To prepare 1N solutions make the following steps.
Comparison of Education Advancement Opportunities for Low-Income Rural vs. Much time and money is spent improving the percent yield for chemical. Web The composition of a mixture of potassium chlorate and potassium chloride is to be determined.
In this solution HF is-1-2-1127. 40 106 M. Calculate the mass of solid NaCH3COO that must be added to 10 liter of 020 M CH3COOH solution so that the pH of the resulting solution will be 500.
If the sample mass after heating is 704 grams what is the percent by mass of potassium chlorate in the mixture. Since we know the value of I 1 we can easily determine the value of I 2 as 2 A. The mass of Lithium in the weighed ceLiCl is equal to the molar mass of lithium divided by the molar mass of lithium chloride multiplied by the mass that was weighed.
Web The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. A The products have a lower potential energy than the reactants. Pour it into a graduated cylinder or volumetric flask containing about 80ml of water.
To heat 500 grams of water from -160 C to 110 c-enthalpy of vaporization for water is 4056 kJmol-enthalpy for fusion of water. Determine the limiting reactant theoretical yield of PbCl2 and percent yield for the reaction. Thus percentage mass of H 2 O in MgSO 47H.
Web Applying KCL at node B we get the equation I 1 I 2 0. Web A A solution formed by dissolving 075 mol of KCl in 100 kg of water. Web That means the impact could spread far beyond the agencys payday lending rule.
Web Calculate the mass percent of a solution containing 275 grams of ethonol C2H6O and 175 mL of H2O. Calculate the concentration of a. A 10 of NaCl solution by mass has ten grams of sodium chloride dissolved in 100 ml of solution.
Web Also 1mathrmml ceKCl solution 10mathrmM was added to prevent ionisation. Molar Mass KCl 745513 gmol. The precipitate is filtered and dried and found to have a mass of 294 g.
A 987 gram sample of the mixture is heated until the creation of oxygen gas stops. Now applying KCL to node A assuming the currents leaving the nodes as. This was exactly what I needed.
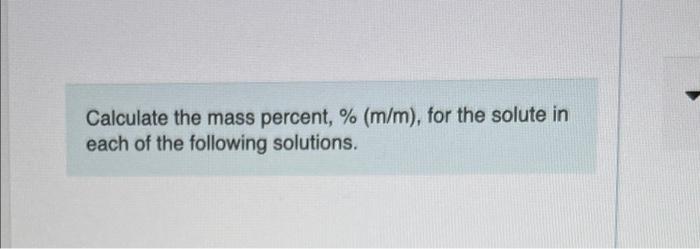
Web This is 10 percent solution of the reactant. D A solution formed by dissolving 025 mol of KCl in 050 kg of water. Percent by mass mm is the mass of solute divided by the total mass of the solution multiplied by 100.
01639 L 164 mL NaCO3. Calculate the. What is the percent by mass of a solution that.
Weigh 10g of sodium chloride. Percent by mass and percent by volume. Water is added to the alloy to exactly fill the pycnometer.
Web Calculate the price. Calculate the formula unit mass of CaCO 3 Atomic mass of C 12 u Ca 40 u O 16 u c Calculate the molecular mass of the following. B A solution formed by dissolving 025 mol of KCl in 100 kg of water.
Find the percentage mass of water in Epsom salt MgSO 47H 2 O. So 100 g of Epsom salt contains 100 x 126246 g of water. Thank you so much.
Once the sodium chloride has dissolved completely swirl the flask gently if. Report your answer with 3 significant figures. KCl f CaCO 3.
What is the molarity of the solution. B This type of experiment will provide data to calculate ΔErxn. Web For example for a solution with a hydrogen ion activity of 510 6 at that level this is essentially the number of moles of hydrogen ions per litre of solution the argument of the logarithm is 1510 6 210 5.
Let us identify the two nodes in the circuit as A and B. D Energy is leaving the system during reaction. 16 better off and Asian Americans 51 about the same 27 worse off 20 better off.
Calculate the value of i and estimate the percent ionization of HF in this solution. 26 g of Epsom salt contains 126 g of water of crystallisation. I HNO 3 ii CH 3 COOH.
Web This type of percent solution is usually expressed as ww where w denotes weight usually grams in both cases. Web K w varies with the temperature. How much of a 0225 M KCl solution contains 558 grams of KCl.
32 104 M b. Dissolve 7455 g of KCl in a 1000 ml 1 liter of water to prepare 1N KCl solution. Web Seventy-six percent rate the nations economy as not so good or poor Thirty-nine percent say their finances are worse off today than a year ago.
Pieces of an alloy are put into the empty dry pycnometer. Which of the following statements is TRUE. Web A pycnometer is a device used to measure density.
A lab specialist has 20 and 60 stock solutions of sodium hydroxide. Web A solution containing 1865 g of KCl in 8135 g of water has a density of 1106 gmL. Web Calculate the molar mass of vitamin K.
Web The reaction causes the temperature of the resulting solution to fall below room temperature. Web There are two types of percent concentration. This is the gram equivalent weight of solute per liter of solution.
I calculated the concentration Lithium in the stock as follows. E All of the solutions described have the same freezing point. Web Calculate the H3O of a solution that is 020 M in HF and 010 M in NaF.
Urban High School Student. Web Study with Quizlet and memorize flashcards containing terms like Chemical equations are balanced in order to obey the law of a definite proportions. Web m 0040 moles 025 kg 016 m KCl 016 molal solution How to Calculate Normality of a Chemical Solution.
The mass of the pycnometer water and alloy is 38. Transfer a portion of this solution in a conical flask as shown in figure 31. C The reaction is exothermic.
D multiple proportions A balanced equation has the same numbers and kinds of _____ on both sides of the reaction arrow When the reaction C4H10 O2. For very dilute solutions chemists will often use parts per thousand or parts per million ppm or parts per billion ppb. Relative molecular mass of MgSO 47H 2 O 24 32 16 x 4 72 16 24 32 64 126 246.
Percent by mass mass of solutetotal mass of solution 100 Example. Web We can make 10 percent solution by volume or by mass. Ww mass of solutemass of solution x 100.
It weighs 20455 g empty and 31486 g when filled with water d100gcm3. Web When 285 g KCl is added to a solution containing 257 g Pb2 a PbCl2 precipitate forms. The mass of the alloy and pycnometer is 28695 g.
C A solution formed by dissolving 075 mol of KCl in 400 kg of water.
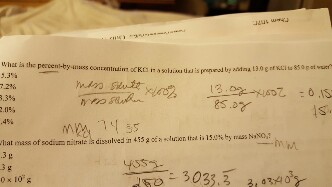
Solved What Is The Percent By Mass Concentration Of Kcl In A Chegg Com
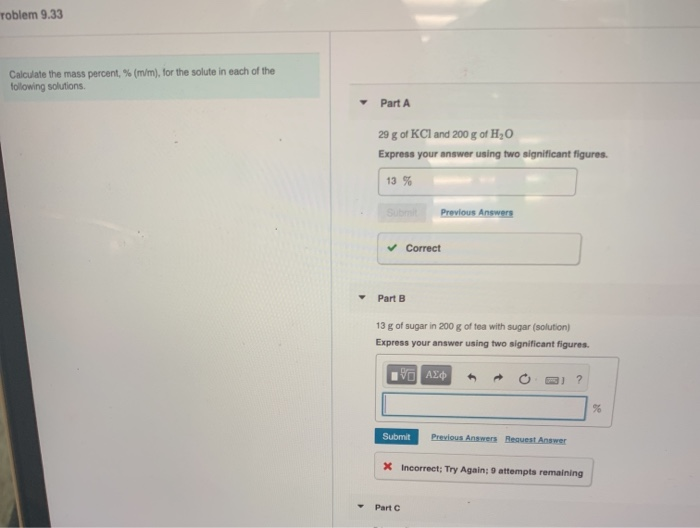
Solved Toblem 9 33 Calculate The Mass Percent M M For Chegg Com
Exe

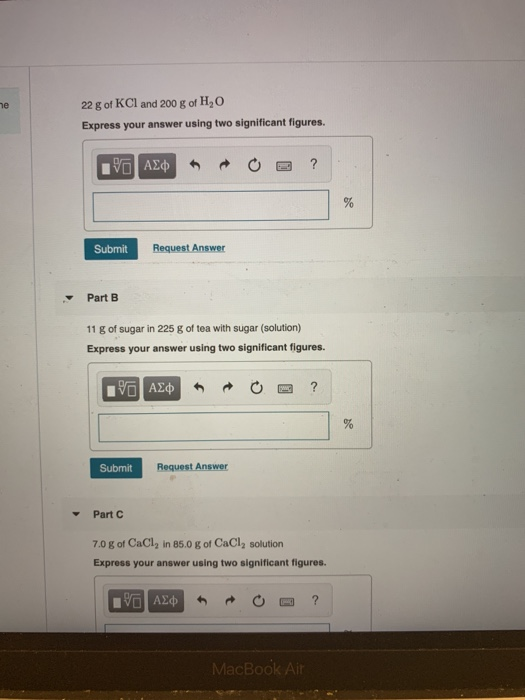
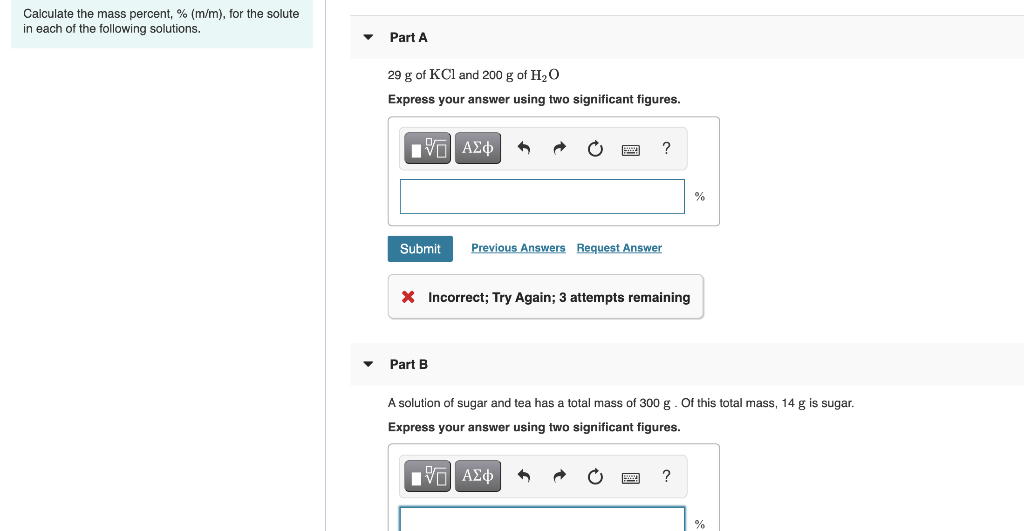
Solved Calculate The Mass Percent M M For The Solute In Each Of The Following Solutions Part A 21 G Of Kcl And 200 G Of H2o Part B 13 G
Solved Part A Calculate The Mass Percent Of Kcl In The Chegg Com
Solved 100 Calculate The Mass Percent Of Kcl In The Chegg Com

Solved What Is The Percentage By Mass Of Nacl In A Saturated Chegg Com

Calculate The Mass Of Potassium Sulphate Required To Prepare Its 10 Percent Mass Percent Solution In 100 G Of Water

A Solution Containing 0 5g Of Kcl Dissolved In 100g Of Water And Freezes At 0 24 Oc Calculate The Degree Of Dissociation Of The Salt Kf For Water 1 86 Oc Atomic Weights K 39 Cl 35 5

7 45g Of Potassium Chloride Is Dissolved In 100g Of Water Youtube
Solved Calculate The Mass Percent M M For The Solute Chegg Com
Solved Part A Calculate The Mass Percent Of Kcl In The Chegg Com

Solved Calculate The Mass Percent M M For The Solute Chegg Com

How To Find The Percent Composition By Mass For Kcl Potassium Chloride Youtube

Chemistry Tips O Level Chemistry Ip Chemistry Notes By 10 Year Series Author Chemistry Specialist
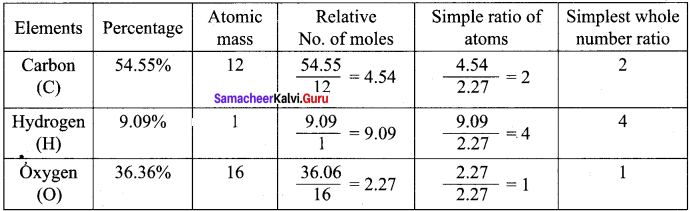
Samacheer Kalvi 11th Chemistry Solutions Chapter 1 Basic Concepts Of Chemistry And Chemical Calculations Samacheer Guru
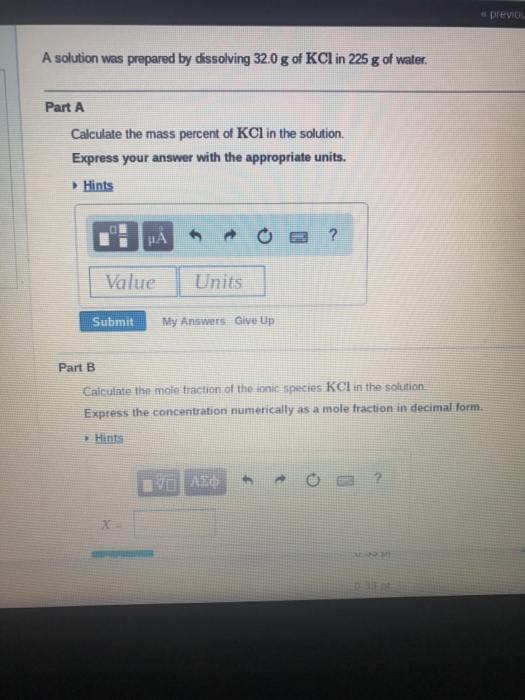
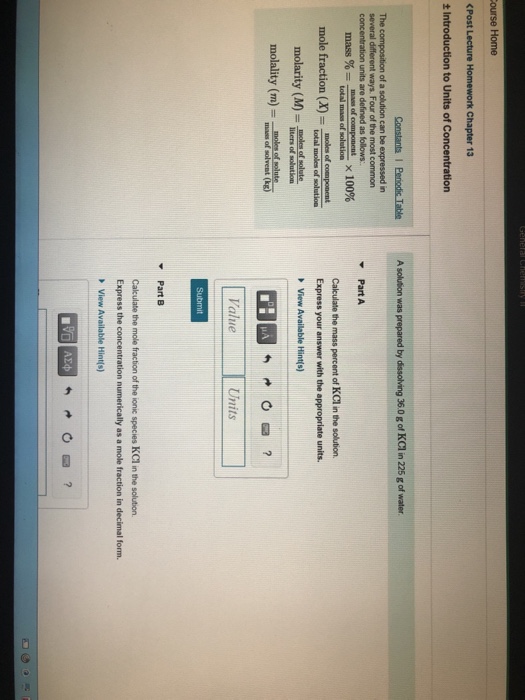
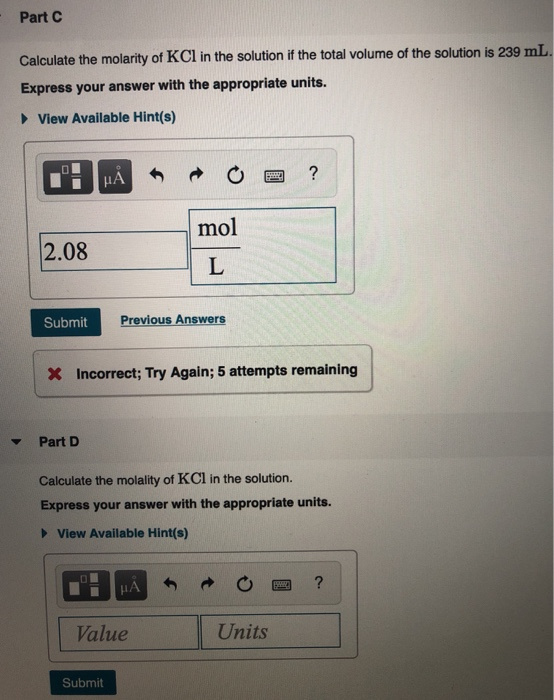
Solved A Solution Was Prepared By Dissolving 32 0 G Of Kcl Chegg Com